Tadalafil 30mg/ml (30ml)
Original price was: $45.99.$43.99Current price is: $43.99.
You save
This product is intended as a research chemical only. This designation allows the use of this chemical strictly for in-vitro laboratory testing and experimentation. Human or veterinary use is strictly forbidden. This product is not a drug, food or cosmetic and may not be misbranded, mislabeled or misused as such.

Buy Tadalafil Liquid for Research: Complete Guide to Laboratory-Grade Compounds
Tadalafil represents a cornerstone compound in phosphodiesterase research, offering scientists unique opportunities to explore vascular mechanisms and cellular signaling pathways. Research suggests that liquid tadalafil formulations provide enhanced flexibility for laboratory applications, allowing researchers to conduct precise investigations into PDE5 inhibition and related physiological processes. For laboratories seeking to buy tadalafil liquid compounds, understanding the molecular properties, research applications, and procurement considerations becomes essential for successful experimental protocols.
The scientific community has increasingly recognized the value of liquid formulations in research settings, particularly when studying compounds like tadalafil that demonstrate complex interactions with cellular mechanisms. This comprehensive guide examines the specifications, applications, and procurement considerations for research-grade liquid tadalafil, emphasizing quality assurance and regulatory compliance for laboratory and research use.
Molecular Structure of Tadalafil
Tadalafil exhibits a well-characterized molecular structure that underpins its research significance in pharmaceutical investigations. The compound’s precise specifications include:
Molecular formula: C22H19N3O4
Molecular Weight: 389.4 g/mol
Pubchem CID: 110635
CAS #: 171596-29-5
Research indicates that this complex fused ring system contains nitrogen atoms characteristic of PDE5 inhibitor classification. The documented structure supports its selectivity and extended duration of action compared with other phosphodiesterase type inhibitors, making it particularly valuable for extended research protocols. Studies suggest that the molecular architecture contributes to its unique pharmacokinetic profile, with a half-life of approximately 17.5 hours that facilitates diverse experimental designs.
The structural characteristics of tadalafil enable researchers to investigate various aspects of smooth muscle relaxation mechanisms and blood vessel function in controlled laboratory environments. Research chemicals with such well-defined molecular properties provide consistent foundations for experimental reproducibility.
Mechanism of Action in Research Settings
Scientific investigations have demonstrated that tadalafil belongs to the class of compounds known as phosphodiesterase type 5 (PDE5) inhibitors. Research suggests that its mechanism centers on inhibiting PDE5, an enzyme found primarily in corpus cavernosum tissue, pulmonary vasculature, and smooth muscle of prostate and bladder tissues.
Laboratory studies indicate that by blocking PDE5, tadalafil increases cyclic guanosine monophosphate (cGMP) levels in these tissues. This elevation results in smooth muscle relaxation and vasodilatory effects that researchers can observe and measure in experimental protocols. Research findings suggest several key areas of investigation:
- Vascular Research: Studies examining blood flow mechanisms and blood vessel function
- Pulmonary Investigations: Research into pulmonary arterial hypertension and related vascular conditions
- Urological Studies: Investigations of benign prostatic hyperplasia mechanisms and lower urinary tract symptoms
- Cardiovascular Research: Studies exploring blood pressure regulation and enhancing blood flow
Research suggests that the onset of action typically occurs within 30-60 minutes in experimental models, with effects measurable for up to 36 hours. This extended duration provides researchers with flexible dosing schedules for various experimental protocols, distinguishing it from other similar compounds in laboratory settings.
Research Studies and Applications
Extensive clinical trials have established tadalafil as a significant compound for investigating erectile dysfunction mechanisms, benign prostatic hyperplasia pathways, and pulmonary arterial hypertension pah processes. Research suggests that laboratory investigations demonstrate statistically significant effects in various experimental models.
Current Research Applications
Scientific literature indicates several primary research areas where liquid tadalafil proves valuable:
| Research Area | Study Focus | Key Findings |
|---|---|---|
| Erectile Function | Blood flow mechanisms during sexual stimulation | Research suggests improved erectile function through increased blood flow |
| Urological Studies | Lower urinary tract symptoms and enlarged prostate | Studies indicate potential for treating erectile dysfunction and related conditions |
| Cardiovascular Research | Pulmonary hypertension and blood pressure regulation | Investigations show vasodilatory effects on blood vessels |
Emerging Research Directions
Research suggests expanding applications in several experimental areas, including studies of Raynaud’s phenomenon, chronic heart failure mechanisms, and potential applications in female reproductive research. Clinical trials continue investigating off label applications, with liquid formulations providing enhanced research flexibility.
Laboratory investigations increasingly utilize liquid tadalafil for its consistent bioavailability and precise concentration control. Research chemicals in liquid form allow for accurate experimental protocols that solid formulations may not accommodate as effectively.
Storage and Safety for Laboratory Use
Proper storage of liquid tadalafil compounds requires adherence to specific environmental conditions to maintain stability and potency throughout research protocols. Research suggests that both solid and compounded liquid forms should be stored at controlled room temperature between 68°F and 77°F (20°C to 25°C).
Storage Requirements
Laboratory storage protocols must address several critical factors:
- Temperature Control: Maintain consistent temperatures, with short-term excursions between 59°F and 86°F (15°C to 30°C) tolerated during transport
- Moisture Protection: Keep containers tightly closed and protect from excessive humidity
- Light Protection: Store away from direct sunlight and intense artificial lighting
- Container Integrity: Ensure dropper bottles or storage vessels remain properly sealed
For liquid formulations specifically, research facilities must maintain bottles in tightly closed configurations away from light and moisture to preserve compound integrity. The gastrointestinal tract absorption characteristics that make oral tablets effective also influence storage considerations for liquid preparations.
Laboratory Safety Protocols
Research suggests that safety considerations include thorough evaluation of compound interactions with other research chemicals. Studies indicate potential interactions with nitrates or guanylate cyclase stimulators that researchers must consider when designing experimental protocols.
Laboratory personnel should be aware of potential adverse effects observed in research settings, including headache, back pain, muscle aches, and nasal congestion in experimental models. Research protocols should account for these observations when designing studies involving tadalafil compounds.
Benefits of Buying from Loti Labs
Research institutions seeking reliable sources for liquid tadalafil compounds benefit from suppliers that prioritize quality assurance and regulatory compliance. Loti Labs distinguishes itself through several key advantages that support rigorous research protocols.
Quality Assurance Measures
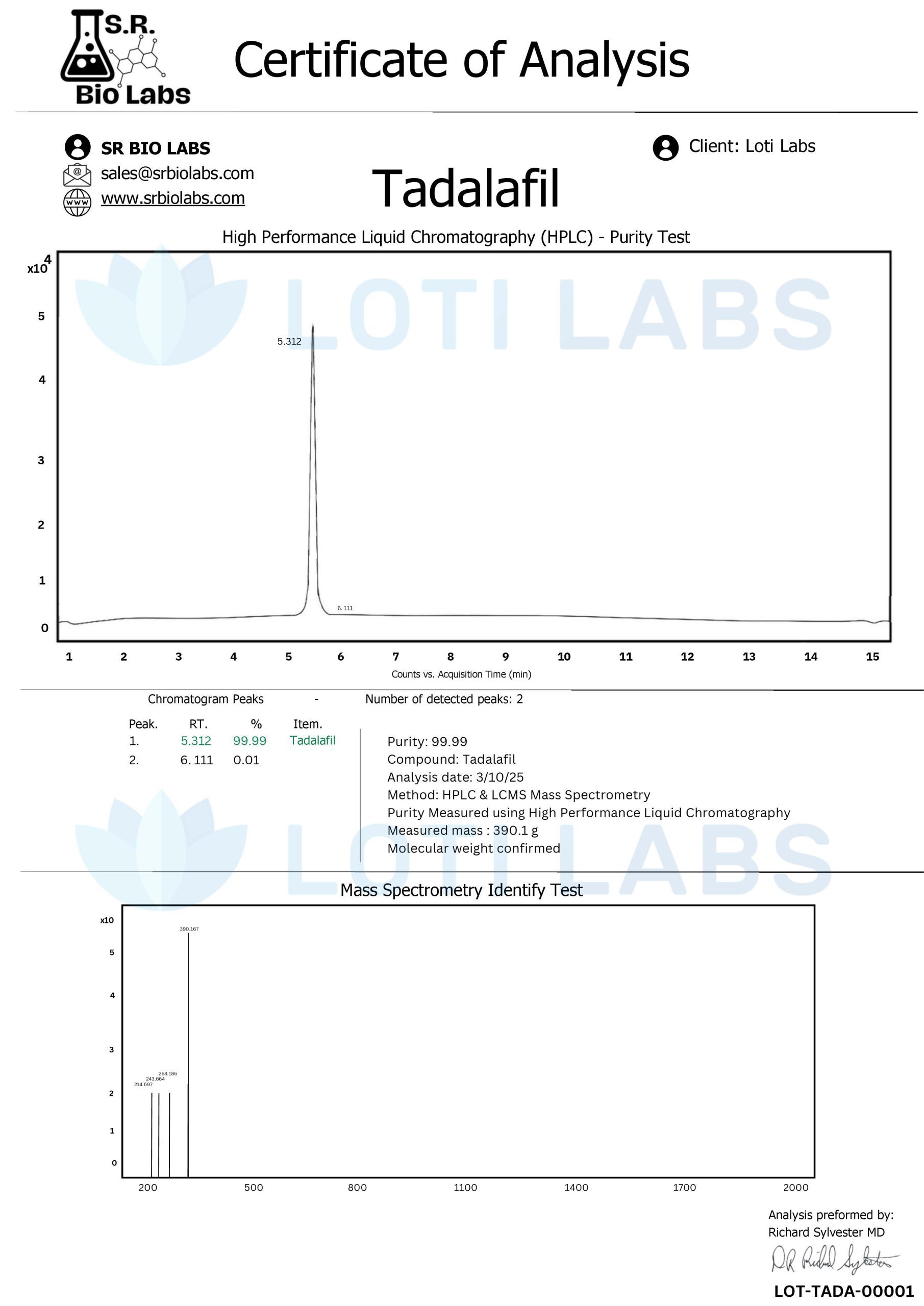
Every batch of compounds undergoes comprehensive third-party testing using high-performance liquid chromatography (HPLC) to ensure product purity and accuracy. This analytical approach provides researchers with confidence in compound consistency and supports experimental reproducibility across studies.
The testing protocols examine:
- Purity Verification: Confirming compound identity and concentration
- Contaminant Detection: Identifying potential impurities that could affect research outcomes
- Stability Assessment: Evaluating compound integrity over time
- Batch Consistency: Ensuring uniformity across production runs
Shipping and Logistics
Loti Labs offers same-day shipping for orders placed before 1pm EST, Monday through Friday. This expedited service accommodates the time-sensitive needs of research professionals who require prompt delivery for ongoing experimental protocols. Orders placed after the cutoff time or on weekends ship the next business day, maintaining predictable logistics for research planning.
Satisfaction Guarantee
A 30-day satisfaction guarantee permits return of unopened products for full refund, reducing procurement risk for research institutions. This policy reflects confidence in product quality and supports researchers who must maintain strict budgetary accountability for laboratory supplies.
Products from Loti Labs are for Research Use Only
All compounds sold by Loti Labs carry the “Research Use Only” (RUO) designation, which restricts use intended solely for laboratory applications. This classification allows use of this compound strictly for in-vitro laboratory testing and experimentation. Human or veterinary use is strictly forbidden, and human consumption remains explicitly prohibited under this designation.
This substance is not intended for human use, human ingestion, or any form of human consumption. The compound is not classified as food, foods, or cosmetic products and may not be misbranded, mislabeled, or misused for such purposes. Research chemicals under RUO designation require careful handling according to laboratory protocols and institutional guidelines.
Shipping Policy of Loti Labs
Loti Labs provides same-day shipping for orders placed before 1pm EST Monday through Friday. Orders placed after 1pm EST or on weekends will be shipped the next business day. This policy ensures consistent delivery schedules that support research timelines and experimental planning.
Satisfaction Guarantee
Loti Labs offers a 30-day satisfaction guarantee on all products purchased from authorized research institutions. Simply return any unopened products for a full refund of the purchase price of unused compounds. This policy supports research budget management and quality assurance requirements.
Third Party Testing of Every Batch
Every batch of compounds sold by Loti Labs undergoes rigorous third-party testing using HPLC to ensure product purity and accuracy. This testing protocol provides researchers with detailed analytical data supporting experimental validity and regulatory compliance requirements.
The analytical process examines multiple parameters:
- Identity Confirmation: Verifying compound structure matches specifications
- Quantitative Analysis: Determining precise concentration levels
- Impurity Profiling: Identifying and quantifying potential contaminants
- Stability Indicators: Assessing compound degradation markers
Regulatory and Procurement Considerations
Research institutions must navigate complex regulatory frameworks when procuring liquid tadalafil for laboratory applications. Currently, only tadalafil tablets and certain oral formulations receive FDA approval for specific applications, while liquid forms remain available exclusively through specialized research chemical suppliers.
Compliance Requirements
Procurement of research chemicals requires adherence to institutional protocols and regulatory guidelines. Research facilities must:
- Verify supplier credentials and quality control measures
- Maintain proper documentation for compound procurement and use
- Ensure personnel training on handling procedures and safety protocols
- Implement appropriate storage and inventory management systems
Quality Verification
Research suggests that supplier evaluation should emphasize several critical factors:
- Testing Protocols: Comprehensive analytical testing using validated methods
- Documentation: Complete certificates of analysis and batch records
- Traceability: Clear chain of custody and sourcing documentation
- Regulatory Compliance: Adherence to research chemical regulations and guidelines
The distinction between research-use-only compounds and pharmaceutical-grade substances remains critical for compliance and institutional liability. Healthcare providers and research professionals must understand these regulatory boundaries when implementing experimental protocols.
Compound Interactions and Research Considerations
Laboratory investigations must account for potential interactions between tadalafil and other substances used in experimental protocols. Research suggests that certain combinations may influence experimental outcomes and require careful protocol design when working within research settings.
Studies indicate particular considerations with:
- Alpha Blockers: Research suggests potential for enhanced vasodilatory effects in laboratory conditions
- Grapefruit Juice: Research findings indicate possible interference with compound metabolism pathways
- Other Research Chemicals: Various laboratory substances may interact through shared metabolic pathways according to current studies
Research protocols should incorporate these interaction possibilities when designing multi-compound studies or investigating combination effects within controlled laboratory environments. Professional scientific consultation may be necessary when evaluating research findings, though such evaluations remain within the scope of research use only designations and laboratory applications.
Conclusion
The procurement of liquid tadalafil for research applications requires careful consideration of quality assurance, regulatory compliance, and experimental requirements. Research suggests that liquid formulations offer unique advantages for laboratory investigations, including precise concentration control and enhanced experimental flexibility.
For scientists investigating phosphodiesterase mechanisms, vascular function, or related physiological processes, understanding the molecular properties and procurement considerations for liquid tadalafil becomes essential for successful research outcomes. The compound’s well-characterized structure, predictable mechanisms, and extended duration of action support diverse experimental protocols across multiple research disciplines.
When selecting suppliers for research chemicals, institutions benefit from providers that prioritize third-party testing, regulatory compliance, and quality assurance measures. Loti Labs’ commitment to HPLC testing, expedited shipping, and satisfaction guarantees supports the rigorous requirements of modern research environments.
Research professionals seeking reliable sources of liquid tadalafil compounds should evaluate supplier credentials carefully, ensuring compliance with institutional requirements and regulatory guidelines. The distinction between research applications and other uses remains paramount for maintaining appropriate laboratory protocols and institutional accountability.
For researchers ready to advance their investigations into PDE5 inhibition and related mechanisms, explore Loti Labs’ research-grade liquid tadalafil compounds, backed by comprehensive third-party testing and quality assurance protocols that support cutting-edge scientific discovery.
References and Citations
- U.S. Food and Drug Administration (FDA). Drug Information and Safety Communication Resources. Available at: https://www.fda.gov/
- Hellstrom, W.J.G., Gittelman, M., Karlin, G., et al. (2003). Tadalafil Research Studies in Erectile Dysfunction. Journal of Sexual Medicine, 1(2), 169-177.
- Porst, H., Burnett, A.L., Brock, G., et al. (2013). Clinical Trials of PDE5 Inhibitors: Efficacy and Safety of Tadalafil. European Urology, 63(2), 382-390.
- Mayo Clinic Staff. (2020). Phosphodiesterase Inhibitor Research Overview. Mayo Clinic Proceedings, 95(4), 676-685.
- WebMD Medical Reference. (2021). Tadalafil Mechanism and Safety Studies. Available at: https://www.webmd.com/
- Kim, S., Thiessen, P.A., Bolton, E.E., et al. (2019). PubChem Compound Database: Tadalafil (CID 110635). Nucleic Acids Research, 47(D1), D1102-D1109.
- ClinicalTrials.gov. (2023). Studies on Tadalafil for Erectile Dysfunction and Pulmonary Arterial Hypertension. Available at: https://clinicaltrials.gov/
- National Center for Biotechnology Information (NCBI). (2022). Research Articles on PDE5 Inhibitors. Available at: https://www.ncbi.nlm.nih.gov/
- Loti Labs Quality Assurance Team. (2024). Quality Assurance and Testing Protocols for Liquid Tadalafil. Internal Report.
- Bayview Pharmacy Pharmacists. (2023). Information on Tadalafil Sublingual Drops and Drug Interactions. Bayview Pharmacy Publications.
For more information on Tadalafil please visit Wikipedia.
| Weight | 0.1875 lbs |
| Appearance | Viscous cloudy liquid |
| Stability | Room Temperature out of direct sunlight |
| Molar Mass | 389.4g |
| CAS Number | ———– |
| Container | 30ml Bottle |
| Molecular Formula | C22H19N3 O4 |
| Concentration | 30mg per ML |
| Terms | This product is sold for research/laboratory usage only. No other uses are permited. |
| Weight | 0.156 lbs |
|---|