Tamoxifen Citrate 20mg/ml (30ml)
$39.99
You save
This product is intended as a research chemical only. This designation allows the use of this chemical strictly for in-vitro laboratory testing and experimentation. Human or veterinary use is strictly forbidden. This product is not a drug, food or cosmetic and may not be misbranded, mislabeled or misused as such.

Buy Tamoxifen Liquid at Loti Labs: Research-Grade SERM Formulations
Research scientists investigating selective estrogen receptor modulator mechanisms require access to precisely formulated compounds that meet stringent laboratory standards. When laboratories seek to buy tamoxifen liquid for experimental protocols, the quality and documentation of the chemical formulation becomes paramount to achieving reliable, reproducible research outcomes. Tamoxifen citrate, widely recognized as a benchmark SERM compound, exhibits complex tissue-specific effects that make it invaluable for studies examining estrogen receptor interactions across various biological systems.
The liquid form of tamoxifen offers researchers enhanced flexibility in experimental design, allowing for precise concentration adjustments and simplified administration protocols compared to traditional solid formulations. This characteristic proves particularly valuable in dose-response studies where accuracy and reproducibility are essential for valid scientific conclusions.
Molecular Structure of Tamoxifen
The chemical foundation of tamoxifen research begins with understanding its precise molecular composition. Research-grade tamoxifen exhibits the following specifications:
Chemical Name: (Z)-2-[4-(1,2-diphenylbut-1-enyl)phenoxy]-N,N-dimethylethanamine
Molecular Formula: C26H29NO·C6H8O7 (as citrate salt)
Molecular Weight: 563.6 g/mol
PubChem CID: 2733526
CAS Number: 54965-24-1
The triphenylethylene core structure represents the critical component responsible for tamoxifen’s selective estrogen receptor modulator activity. The (Z)-isomer configuration proves essential for antagonistic effects at estrogen receptors, a detail of significant importance for laboratory researchers examining receptor binding mechanisms. When scientists buy tamoxifen liquid from reputable suppliers, they receive formulations that maintain this specific isomeric form, ensuring consistent experimental results across research protocols.
The citrate salt formulation enhances solubility characteristics while providing stability advantages in aqueous solutions. This molecular arrangement allows the compound to remain dissolved in various solvents commonly used in laboratory settings, including ethanol, methanol, and water-based solutions designed for specific experimental requirements.
Mechanism of Action
Tamoxifen functions as a selective estrogen receptor modulator, demonstrating the ability to act as both an antagonist and agonist depending on the target tissue environment. In breast tissue, the compound primarily exhibits antagonistic effects, competitively binding to estrogen receptors and preventing natural estrogen from initiating cellular responses. This tissue-specific action makes tamoxifen particularly valuable for researchers studying hormone-dependent cellular mechanisms and breast cancer cell biology.
The liquid formulation provides researchers with enhanced precision in experimental dosing protocols. Unlike solid forms that may vary in dissolution rates, liquid tamoxifen delivers consistent bioavailability in in vivo studies and uniform concentration distribution in cell culture applications. Research suggests that this consistency proves crucial for establishing reliable dose-response relationships in experimental settings.
Laboratory studies examining the active metabolite formation of tamoxifen have utilized liquid formulations to better control pharmacokinetic variables. The compound undergoes hepatic metabolism, with the liver processing tamoxifen into various metabolites that may exhibit different receptor binding affinities. Researchers investigating these metabolic pathways benefit from the precise dosing control that liquid formulations provide.
Additionally, tamoxifen demonstrates varying effects across different tissues, exhibiting partial agonist activity in bone tissue while maintaining antagonistic effects in breast tissue. This tissue selectivity has encouraged extensive research into SERM mechanisms and has led to investigations of tamoxifen’s role in bone density studies and endometrial tissue research.
Research Studies
Tamoxifen represents one of the most extensively studied SERM compounds in scientific literature, with research applications spanning multiple biological systems and experimental models. Laboratory investigations have utilized tamoxifen in both in vitro cell culture studies and in vivo animal models to examine estrogen receptor signaling pathways and cellular response mechanisms.
Research-grade tamoxifen solutions are formulated in various solvents to optimize experimental conditions. Scientists studying breast cancer cells often use tamoxifen formulations in DMSO or ethanol to achieve desired working concentrations in culture media. The liquid form allows for accurate serial dilutions and consistent experimental conditions across multiple research protocols.
Transgenic research has incorporated tamoxifen extensively in Cre-Lox recombination systems, where the compound serves to activate Cre recombinase activity in conditional knockout mouse models. These studies require precise timing and dosing, making liquid tamoxifen formulations particularly valuable for achieving reproducible experimental outcomes. The ability to administer exact concentrations ensures that gene activation occurs at predetermined time points, critical for developmental biology research.
Cancer research has utilized tamoxifen to investigate apoptosis mechanisms in hormone-dependent tumor models. Studies examining cellular response pathways have demonstrated that tamoxifen treatment can influence cell cycle progression and programmed cell death in specific cancer cell lines. Research suggests that these effects may vary depending on the cellular environment and concurrent treatment conditions.
Prevention studies in high risk populations have provided valuable data regarding tamoxifen’s potential protective effects against developing breast cancer. Laboratory models examining these protective mechanisms have utilized liquid tamoxifen formulations to establish optimal treatment protocols and examine dose-dependent responses.
Storage and Safety
Proper storage protocols ensure that tamoxifen liquid maintains its chemical integrity and research-grade quality throughout its shelf life. The recommended storage conditions require maintaining the formulation at room temperature (20-25°C) in tightly sealed containers protected from direct light and moisture exposure.
Temperature control proves critical for preserving tamoxifen’s molecular stability. Exposure to freezing temperatures may cause precipitation or crystallization that could alter the compound’s solubility characteristics, while excessive heat exposure may promote degradation reactions that reduce the active compound concentration. Research facilities should establish standard operating procedures for chemical storage that include regular temperature monitoring and appropriate containment measures.
Light protection represents another essential storage consideration, as tamoxifen exhibits photosensitivity that can lead to degradation over time. Loti Labs packages tamoxifen liquid in light-resistant containers specifically designed to prevent photodegradation during storage and handling. Laboratories should continue this protection by storing opened containers in dark environments and minimizing light exposure during experimental procedures.
The FDA has issued important safety considerations regarding tamoxifen that research facilities should incorporate into their safety protocols. These include awareness of potential uterine malignancies and thromboembolic events that have been associated with tamoxifen in clinical studies. While these considerations apply primarily to therapeutic use, research facilities should maintain appropriate safety measures and documentation when handling tamoxifen compounds.
Laboratory personnel should follow established protocols for handling research chemicals, including the use of appropriate personal protective equipment and secure storage procedures. Contact with skin or mucous membranes should be avoided, and any questions regarding proper handling procedures should be directed to qualified safety personnel.
Benefits of Buying from Loti Labs
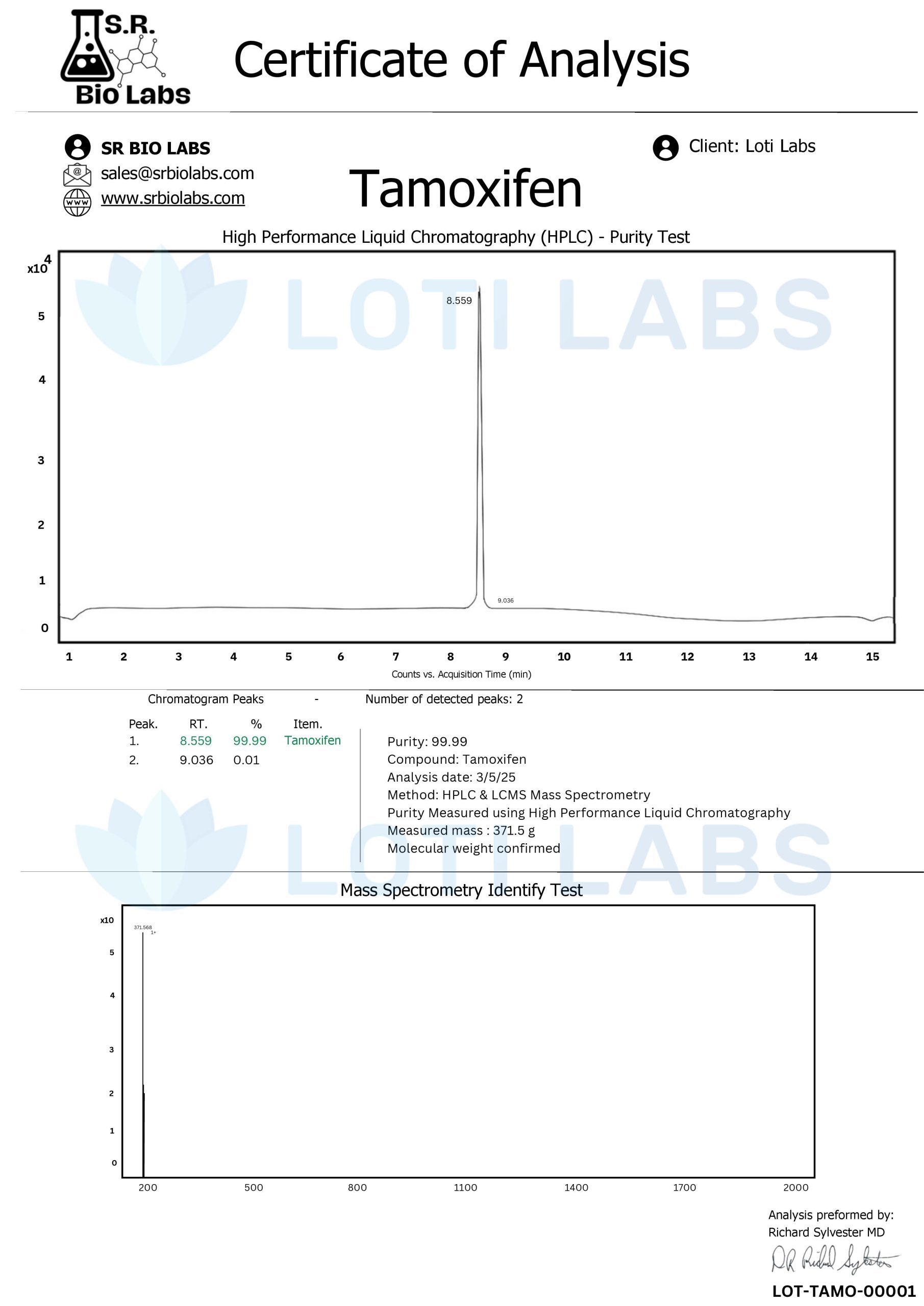
Loti Labs provides pharmaceutical-grade tamoxifen citrate liquid formulated to exceed standard research requirements. The company’s tamoxifen formulations maintain HPLC-verified purity levels exceeding 99%, ensuring that researchers receive compounds suitable for the most demanding experimental protocols. This level of purity proves essential for studies where even minor impurities could influence experimental outcomes or compromise data reliability.
The standard concentration of 20 mg/ml provides researchers with a convenient working strength that can be easily diluted to achieve desired experimental concentrations. For laboratories requiring different concentrations, Loti Labs offers custom formulation services that can produce tamoxifen liquid at specific concentrations tailored to individual research requirements. This flexibility allows research teams to optimize their experimental protocols without being constrained by standard formulation limitations.
Each batch of tamoxifen liquid includes comprehensive certificates of analysis that document purity profiles, concentration verification, and stability parameters. These certificates provide researchers with the documentation necessary for regulatory compliance and experimental validation. The detailed analytical data supports research integrity and provides confidence in experimental results.
Quality control measures extend beyond initial formulation to include packaging considerations designed to preserve product integrity. Tamoxifen formulations are packaged in appropriate light-resistant containers that prevent degradation during storage and shipping. This attention to packaging details ensures that researchers receive products that maintain their specified characteristics from production through experimental use.
The company’s focus on research-grade quality distinguishes its products from formulations intended for other applications. This specialization ensures that researchers receive compounds optimized for laboratory use, with purity standards and documentation appropriate for scientific research requirements.
Products from Loti Labs are for Research Use Only
All tamoxifen liquid products sold by Loti Labs are designated exclusively for research applications and are not intended for human or veterinary therapeutic use. This designation aligns with regulatory frameworks governing laboratory chemicals and ensures that products are properly classified for their intended use in scientific research settings.
The research-use-only status provides laboratories with access to high-quality chemical formulations while maintaining clear boundaries regarding appropriate use. This classification allows researchers to utilize tamoxifen in controlled experimental environments without the regulatory constraints associated with pharmaceutical products intended for therapeutic applications.
Research institutions must ensure that tamoxifen compounds are used exclusively for laboratory applications and are not confused with pharmaceutical-grade products intended for clinical use. Proper labeling and storage protocols help maintain this distinction and prevent any misunderstanding regarding the product’s intended use.
The product catalog includes detailed specifications and handling information that support proper use in research environments. Scientists requiring assistance with product selection or application questions are encouraged to contact Loti Labs’ technical support team for guidance on appropriate use protocols.
Shipping Policy of Loti Labs
Loti Labs maintains efficient shipping protocols designed to ensure product integrity during transit while meeting the time-sensitive needs of research institutions. Orders placed before 1 PM EST Monday through Friday receive same-day processing and shipping, allowing researchers to maintain project timelines and minimize experimental delays.
Orders received after the daily cutoff time or on weekends are processed and shipped on the next business day, ensuring consistent handling protocols regardless of order timing. This standardized approach helps research facilities plan their procurement schedules and anticipate delivery timeframes for ongoing projects.
All tamoxifen liquid formulations receive careful packaging designed to maintain chemical stability during shipping. The packaging protocols consider temperature control, light protection, and physical protection to ensure that products arrive in optimal condition for immediate use in research applications.
Research institutions can submit purchase orders through established procurement channels, with appropriate documentation and credentials verified through secure ordering systems. This process accommodates institutional purchasing requirements while maintaining security and compliance standards.
Custom formulations requiring specialized testing protocols typically require 7-14 days for production and quality verification. This timeframe allows for complete analytical testing and documentation preparation, ensuring that custom products meet the same quality standards as standard formulations.
Satisfaction Guarantee
Loti Labs offers a comprehensive 30-day satisfaction guarantee on all products, allowing researchers to return unopened products for a full refund if the formulation does not meet their experimental requirements. This guarantee provides research institutions with confidence in their procurement decisions and reduces the risk associated with trying new suppliers or formulations.
The satisfaction policy proves particularly valuable for research facilities ordering bulk quantities or establishing long-term supply relationships. Institutions can evaluate product quality and suitability for their specific research applications while maintaining the option to return products that do not meet their expectations.
All products undergo rigorous quality control measures before shipping, including analytical verification and stability testing. This comprehensive testing protocol ensures that researchers receive products that meet or exceed specified quality parameters, reducing the likelihood of product-related experimental complications.
Research facilities requiring specialized guarantee terms for bulk purchases or extended studies may contact customer service for customized arrangements. The company’s flexibility in addressing unique institutional needs demonstrates its commitment to supporting diverse research requirements.
The guarantee policy covers only unopened products, emphasizing the importance of initial product evaluation before use in experimental protocols. This policy protects both the researcher and the supplier while ensuring that returned products maintain their integrity for potential resale.
Third-Party Testing of Every Batch
Every batch of tamoxifen liquid undergoes comprehensive third-party HPLC analysis to verify purity and concentration accuracy. This independent testing protocol ensures that products meet specified parameters and provides researchers with confidence in the analytical data supporting their experimental work.
The third-party testing approach eliminates potential conflicts of interest in quality assessment and provides additional validation of product specifications. Independent laboratories conduct these analyses using calibrated instruments and validated methods, ensuring that results meet industry standards for analytical accuracy.
Certificates of analysis accompanying each batch document purity levels, concentration verification, and the absence of significant contaminants. This documentation proves essential for researchers working under Good Laboratory Practice (GLP) guidelines or other regulatory frameworks requiring detailed analytical documentation.
Batch-to-batch consistency represents a critical factor in maintaining experimental reproducibility across extended research projects. The consistent testing protocols and quality standards ensure that researchers can rely on uniform product characteristics regardless of when their supply is purchased or which batch they receive.
Research facilities can request specific testing parameters for custom formulations, allowing for tailored analytical protocols that address unique experimental requirements. This flexibility ensures that specialized research applications receive appropriate analytical support while maintaining the company’s standard quality assurance protocols.
References and Citations
The extensive research literature supporting tamoxifen’s role as a selective estrogen receptor modulator provides researchers with a wealth of background information for experimental design and interpretation. Scientific publications have documented tamoxifen’s mechanisms of action across various tissue types and experimental models, providing valuable context for new research applications.
Clinical studies examining tamoxifen’s effects in breast cancer treatment and prevention have generated substantial data regarding the compound’s pharmacology and tissue-specific activities. While these studies focus on therapeutic applications, they provide important mechanistic insights that inform basic research applications and experimental protocol development.
Laboratory studies investigating tamoxifen’s role in estrogen receptor signaling pathways have utilized various experimental models to characterize the compound’s molecular mechanisms. These investigations have contributed to understanding SERM selectivity and have identified key factors influencing tissue-specific responses to tamoxifen treatment.
Research examining tamoxifen’s metabolic pathways and the activity of its active metabolite has provided insights into factors that may influence experimental outcomes. Understanding these metabolic considerations helps researchers design appropriate experimental controls and interpret results in the context of compound pharmacokinetics.
Studies utilizing tamoxifen in transgenic mouse models have established protocols for conditional gene expression systems that rely on tamoxifen-inducible Cre recombinase activity. These applications have expanded tamoxifen’s utility in developmental biology and genetics research, demonstrating the compound’s versatility across diverse research disciplines.
When researchers buy tamoxifen liquid from Loti Labs, they gain access not only to high-quality research compounds but also to technical support and documentation that facilitates successful experimental outcomes. The combination of product quality, analytical documentation, and customer support creates a comprehensive resource for scientists investigating SERM mechanisms and related research questions. For additional information or assistance with product selection, research facilities are encouraged to contact Loti Labs directly to discuss their specific experimental requirements and ensure optimal product selection for their research applications.
References
- Jordan VC. Tamoxifen: a pioneering medicine in breast cancer treatment and prevention. Nat Rev Drug Discov. 2003;2(3):205-213. doi:10.1038/nrd1035
- Howell A, Osborne CK, Morris C, Wakeling AE. ICI 182,780 (Faslodex): development of a novel antiestrogen. Cancer. 2000;89(4):817-825. doi:10.1002/(SICI)1097-0142(20000815)89:4<817::AID-CNCR21>3.0.CO;2-8
- McDonnell DP, Wardell SE. The molecular mechanisms underlying the pharmacological actions of ER modulators: implications for new drug development in breast cancer. Curr Opin Pharmacol. 2010;10(6):620-628. doi:10.1016/j.coph.2010.08.004
- Fisher B, Costantino JP, Wickerham DL, et al. Tamoxifen for prevention of breast cancer: report of the National Surgical Adjuvant Breast and Bowel Project P-1 Study. J Natl Cancer Inst. 1998;90(18):1371-1388. doi:10.1093/jnci/90.18.1371
- Loti Labs. Tamoxifen Liquid Product Information and Safety Data. Accessed 2024.
- U.S. Food and Drug Administration. Tamoxifen (Nolvadex) Drug Safety Information. Accessed 2024.
- Yildiz A, Guleryuz S, Ankerst DP, et al. Protein kinase C inhibition in the treatment of mania: a double-blind, placebo-controlled trial of tamoxifen. Arch Gen Psychiatry. 2008;65(3):255-263. doi:10.1001/archgenpsychiatry.2007.29
- Jordan VC. Selective estrogen receptor modulation: concept and consequences in cancer. Cancer Cell. 2004;5(3):207-213. doi:10.1016/S1535-6108(04)00049-8
- Bayview Pharmacy. Tamoxifen Citrate 6 mg/ml Oral Liquid – Compounded Solution. Accessed 2024.
- Krum SA, Miranda-Carboni GA, Hauschka PV, et al. Estrogen protects bone by inducing Fas ligand in osteoblasts to regulate osteoclast survival. EMBO J. 2008;27(3):535-545. doi:10.1038/sj.emboj.7601976
For more information on Tamoxifen Citrate visit Wikipedia.
| Weight | 0.1875 lbs |
| Appearance | Viscous cloudy liquid |
| Stability | Room Temperature out of direct sunlight |
| Molar Mass | 563.7 |
| CAS Number | 54965-24-1 |
| Container | 30ml Bottle |
| Molecular Formula | C32H37NO8 |
| Concentration | 20mg per ML |
| Terms | This product is sold for research/laboratory usage only. No other uses are permited. |
| Weight | 0.156 lbs |
|---|