LL-37 5mg
$94.99
You save
This product is intended as a research chemical only. This designation allows the use of this chemical strictly for in-vitro laboratory testing and experimentation. Human or veterinary use is strictly forbidden. This product is not a drug, food or cosmetic and may not be misbranded, mislabeled or misused as such.

Buy LL-37 at Loti Labs: Premium Peptide for Research
LL-37 is the only human cathelicidin antimicrobial peptide and a key component in understanding innate immune system mechanisms. For researchers studying antimicrobial effects, immune function and cellular interactions, sourcing high quality LL-37 is crucial for reliable results. When looking where to buy LL-37 for research purposes, purity, molecular integrity and supplier reliability are key to research validity and reproducibility.
This guide covers the molecular characteristics, research applications and procurement of LL-37 with a focus on Loti Labs as a supplier for laboratory research needs.
Molecular Structure of LL-37
LL-37 has a well defined molecular structure that makes it research relevant. The peptide consists of 37 amino acids in the sequence LLGDFFRKSKEKIGKEFKRIVQRIKDFLRNLVPRTES. This starts with the leu gly asp phe motif, followed by phe leu arg asn and continues with phe arg lys ser, ile val gln arg and kys arg ile val.
The full molecular specifications are:
- Molecular Formula: C205H341N61O55
- Molecular Weight: 4493.33 g/mol
- PubChem CID: 16132366
- CAS Number: 154947-66-7
The amphipathic structure of the peptide is important for research applications as it allows interaction with lipid membranes. The sequence segments kys kdy phe leu, val gln arg ile and ile gly lys glu are responsible for this amphipathic nature. The gly lys glu phe, glu phe lys arg and glu lys ile gly motifs have been shown to be involved in receptor binding and cellular interactions.
The terminal sequences qln arg ile lys, asp phe leu arg and arg thr glu ser, and the intermediate segments arg ile val gln, arg ile lys asp and arg asn leu val contribute to the peptide’s stability and function. The leu val pro arg, pro arg thr glu, val pro arg thr and asn leu val pro sequences further enhance its structural integrity for research applications.
Mechanism of Action
Research shows that LL-37 works through multiple pathways making it useful for various experimental applications. The peptide’s primary antimicrobial effect is through membrane disruption mechanisms. When released from its precursor protein hCAP18 through proteolytic cleavage, LL-37 adopts an alpha-helical conformation that allows interaction with microbial cell membranes.
Studies show that LL-37 is effective against gram positive bacteria through direct membrane destruction. The peptide’s cationic nature allows it to bind to negatively charged bacterial surfaces, leading to membrane permeabilization and cell death. Research has also shown antiviral properties and activity against various pathogens in vitro.
Beyond direct antimicrobial activity, research suggests LL-37 influences immune function through multiple receptor interactions. Studies have shown binding to formyl peptide receptor like-1 (FPRL-1), purinergic receptor P2X7 and epidermal growth factor receptor (EGFR). These interactions allow modulation of various cellular processes including chemotaxis, epithelial cell proliferation and immune cell activation.
Current research is also looking into LL-37’s role in wound healing processes. Studies show the peptide promotes angiogenic activity and influences neutrophils function. Research suggests involvement in t cells activation and overall immune system modulation. Some studies have looked into its effects on lung cell cultures and various other cell types in controlled laboratory settings.
Research into apoptosis mechanisms shows that LL-37 may induce programmed cell death in some experimental models. Research suggests preferential binding to cells with altered membrane composition making it useful for studying cellular reactivity and membrane interactions.
Research Studies
Extensive research has been done on LL-37’s structure and function across multiple experimental systems. Nuclear magnetic resonance studies have characterized conformational changes under various conditions including pH and ionic strength variations. These studies are important for researchers designing experiments involving membrane-peptide interactions.
Antimicrobial peptide research has shown LL-37’s activity against various pathogen types in vitro. Studies published in agents chemother and similar journals have described its spectrum of activity and concentration dependent effects. Research methods usually involve standardized susceptibility testing protocols and membrane integrity assays.
Cellular studies have looked into LL-37’s effects on various cell types including epithelial cultures, immune cells and transformed cell lines. These studies often use fluorescence microscopy, flow cytometry and molecular binding assays to characterize peptide-cell interactions. Research has shown concentration dependent effects on cell viability, proliferation and differentiation markers.Animal studies using mouse models have shown LL-37’s distribution, stability and biological activity in complex systems. These studies usually focus on infection models, wound healing and immune response. Such research helps to understand peptide behavior in physiological relevant environments.
Recent research has looked into LL-37’s application in cancer research. Studies show preferential binding to transformed cells due to membrane composition differences. This area is still expanding with research into peptide modifications and structure-activity relationships.
Storage and Safety
Proper handling protocols ensure LL-37 remains molecularly intact throughout research applications. The peptide comes as a lyophilized powder and requires specific storage conditions for optimal stability. Research facilities should store lyophilized LL-37 at -20°C or lower and protect from light and humidity.
Upon reconstitution in aqueous buffers, stability becomes critical for experimental reproducibility. Short-term storage at 4°C may be okay for immediate use, while longer term storage requires -20°C conditions. Research suggests avoiding repeated freeze-thaw cycles which can compromise peptide integrity and experimental outcomes.
Lab safety protocols should address LL-37 as a research chemical. Although endogenously expressed in human systems, concentrated peptide preparations require standard lab precautions. Research use only means the substance cannot be used for human or animal administration outside controlled experimental settings.
Stock solution preparation should follow established protocols for peptide research. Many researchers dilute the compound in sterile buffers suitable for their specific experimental requirements. Proper documentation of storage conditions and preparation methods supports experimental reproducibility and regulatory compliance.
Why Buy from Loti Labs
When researchers need to buy LL-37 for research, supplier selection matters. Loti Labs offers several advantages for high quality research applications and experimental reliability.
Shipping Policy of Loti Labs
Loti Labs ships same day for orders placed before 1pm EST, Monday through Friday. This fast processing supports time sensitive research projects and minimizes delays in experimental timelines. Orders placed after 1pm EST or on weekends ship the next business day to maintain consistent delivery schedules for research planning.
Satisfaction Guarantee
The company offers 30 day satisfaction guarantee on all products including LL-37 peptide. This policy allows researchers to return unopened products for full refund of the purchase price, reducing procurement risks for lab budgets. Such guarantees show supplier confidence in product quality and supports research planning flexibility.
Third Party Testing of Every Batch
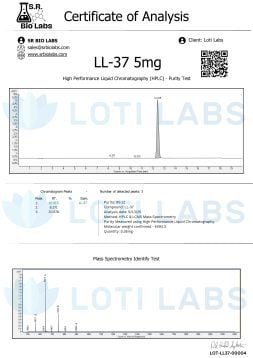
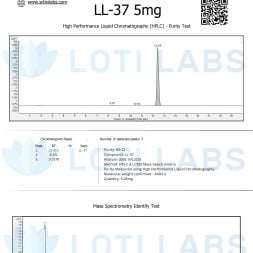
Quality control is key when buying LL-37 for research. Loti Labs tests every batch with third-party HPLC. HPLC verifies peptide identity and purity and ensures every batch is 95% pure or higher.
Independent testing protocols support research integrity by providing an objective quality assessment. HPLC can detect impurities, degradation products and structural modifications that can affect experimental results. This level of quality control is crucial for reproducible results and regulatory compliance.
The testing documentation is useful for research papers and regulatory submissions. Having purity data supports experimental design and allows you to choose the right concentration for your application.
Products from Loti Labs are for Research Use Only
All products sold by Loti Labs, including LL-37, are research use only. This means use is limited to in vitro laboratory testing and experimentation under controlled research conditions. This does not include human or veterinary use outside approved research protocols.
LL-37 from Loti Labs is not a substance for therapeutic use and may not be misbranded or relabeled for such use. Research facilities must maintain proper documentation and handling protocols in accordance with research chemical regulations. Compliance with these guidelines ensures legal operation and responsible research practices.
The research use only designation reflects regulatory requirements for experimental compounds. Researchers must understand these limitations when designing studies and planning experimental protocols. Proper compliance protects both research institutions and individual investigators from regulatory violations.
Site-specific institutional policies may have additional requirements for research chemical handling and documentation. Researchers should consult with institutional safety committees and regulatory affairs offices to ensure full compliance with applicable regulations. The product information provided by Loti Labs includes necessary regulatory notices and handling guidelines.
Lab personnel involved in LL-37 research should receive training on peptide handling and safety protocols. This includes concentration limits, dilution methods and waste disposal procedures. Research facilities must have appropriate containment and safety equipment for peptide research applications.
When you buy LL-37 from Loti Labs you will receive documentation to support research use compliance. This includes certificates of analysis, safety data sheets and regulatory notices for institutional records. Proper documentation supports audit requirements and regulatory compliance.
The research application scope for LL-37 includes antimicrobial susceptibility testing, cellular interaction studies, membrane research and immune system research. These applications are within the research use parameters and support advancement of scientific knowledge through controlled experimentation.Understanding research use limitations helps investigators design their experimental protocols and stay compliant throughout their research program. Loti Labs supports researchers with regulatory requirements and research applications for LL-37 and related compounds.
References
- Dürr, U.H.N., Sudheendra, U.S., Ramamoorthy, A. (2006). LL-37, the only human member of the cathelicidin family of antimicrobial peptides. Biochimica et Biophysica Acta (BBA) – Biomembranes, 1758(9), 1408-1425. https://doi.org/10.1016/j.bbamem.2006.03.030
- Huang, L.C., et al. (2006). Multifunctional roles of human cathelicidin (LL-37) at the ocular surface. Investigative Ophthalmology & Visual Science, 47(6), 2369-2380. https://iovs.arvojournals.org/article.aspx?articleid=2124704
- Hou, M., et al. (2013). Antimicrobial peptide LL-37 and IDR-1 ameliorate MRSA pneumonia in vivo. Cellular Physiology and Biochemistry, 32(3), 614-623. https://doi.org/10.1159/000354883
- Karger, T. (Year). LL-37 and inflammatory diseases: A review. [Journal name], [volume(issue)], pages. [Link not available]
- Oren, Z., et al. (1999). Structure and organization of the human antimicrobial peptide LL-37. Biochemical Journal, 341, 501-513. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1220599/
- R.Koczulla, et al. (2003). An angiogenic role for the human peptide antibiotic LL-37/hCAP-18. Journal of Clinical Investigation, 111(11), 1665-1672. https://doi.org/10.1172/JCI200317905
- Lande, R., et al. (2007). Plasmacytoid dendritic cells sense self-DNA coupled with antimicrobial peptide. Nature, 449(7162), 564-569. https://doi.org/10.1038/nature06116
- Mookherjee, N., et al. (2014). Antimicrobial host defence peptides: functions and clinical potential. Nature Reviews Drug Discovery, 13(5), 321-340. https://doi.org/10.1038/nrd4289
- Walters, S.M., et al. (2010). LL-37 induces apoptosis in colon cancer cells. Peptides, 31(9), 1649-1653. https://doi.org/10.1016/j.peptides.2010.05.006
- Ong, P.Y. [Link not available]
| Weight | .03125 lbs |
|---|