GnRH (Triptorelin) 100mcg
$29.99
You save
This product is intended as a research chemical only. This designation allows the use of this chemical strictly for in-vitro laboratory testing and experimentation. Human or veterinary use is strictly forbidden. This product is not a drug, food or cosmetic and may not be misbranded, mislabeled or misused as such.

Buy Triptorelin at Loti Labs: Premium GnRH Agonist for Research Applications
Researchers studying gonadotropin releasing hormone pathways need access to high purity synthetic analogues for reproducible results. Triptorelin, a potent gnrh agonist, is a game changer in hormone research because of its increased stability and prolonged biological activity compared to the native molecule. When you buy gnrh triptorelin from Loti Labs you get a research grade compound that meets the high purity standards required for scientific research.
This synthetic analogue of gonadotropin releasing hormone gnrh has become an essential tool in endocrinology research, allowing researchers to study hormone dependent mechanisms with unprecedented precision. The compound’s unique molecular modifications provide extended plasma half life beyond that of natural gonadotropin releasing hormone, making it very useful for studies that require sustained hormonal modulation.
Molecular Structure of Triptorelin
Triptorelin has a precisely engineered peptide sequence: Pyr-His-Trp-Ser-Tyr-D-Trp-Leu-Arg-Pro-Gly-NH2. This sequence is a strategic modification of the natural gonadotropin releasing hormone agonist structure, where the substitution of D-Tryptophan at position 6 increases both potency and stability.
The chemical formula C64H82N18O13 corresponds to a molecular weight of 1311.5 g/mol, with the compound catalogued under PubChem CID 638821 and CAS number 57773-63-4. The only change from the native gonadotropin sequence is the incorporation of the D-isomer of tryptophan, which confers resistance to enzymatic degradation and extends the compound’s half-life in biological systems.
| Property | Specification |
|---|---|
| Peptide Sequence | Pyr-His-Trp-Ser-Tyr-D-Trp-Leu-Arg-Pro-Gly-NH2 |
| Molecular Formula | C64H82N18O13 |
| Molecular Weight | 1311.5 g/mol |
| PubChem CID | 638821 |
| CAS Number | 57773-63-4 |
The sequence breakdown shows the strategic positioning: trp ser tyr d trp forms the core modification region, while tyr d trp leu and trp leu arg pro maintain the binding characteristics. The terminal arg pro gly nh2 segment preserves the receptor interaction domain that enables the compound’s agonistic effect.
Mechanism of Action
Triptorelin acts as a hormone releasing hormone agonist by binding to gonadotropin releasing hormone receptors in the anterior pituitary. Upon initial administration the compound causes a surge in both luteinizing hormone and follicle stimulating hormone release, demonstrating its agonistic properties.
Research shows triptorelin controls release of gonadotropins luteinizing hormone and follicle stimulating hormone with high efficiency. Studies have shown approximately 13-fold increased luteinizing hormone releasing activity and 21-fold enhanced follicle stimulating hormone release compared to the endogenous hormone. This increased potency is due to the compound’s resistance to peptidase degradation and improved receptor binding.
The mechanism is biphasic. Initially constant stimulation of pituitary receptors causes increased hormone release. However continuous exposure causes receptor desensitization and downregulation and after approximately 3-4 weeks pituitary secretion of both luteinizing hormone and follicle stimulating hormone decreases.
This dual phase action makes the gnrh agonist triptorelin very useful for research applications that require either acute hormonal stimulation or chronic suppression protocols. The compound has complete absorption after administration, with a volume of distribution of approximately 30-33L in experimental models.
Research Studies
Oncological Research Applications
Triptorelin has shown utility in advanced prostate cancer research models through androgen deprivation mechanisms. Research has shown that the compound can reduce testosterone levels to castrate ranges similar to surgical castration in experimental protocols. Studies in metastatic prostate cancer have shown that triptorelin therapy produces oncological outcomes similar to other gnrh agonists like leuprolide acetate.
The compound’s efficacy in prostate cancer research is due to its ability to halt hormone dependent tumor progression by creating a hypogonadal state. Comparative studies between triptorelin and surgical castration show equivalent effectiveness in experimental models, although the time to achieve castrate testosterone levels may vary slightly from other hormone releasing hormone agonist compounds.
Pediatric Endocrinology Research
Research in central precocious puberty models has shown triptorelin’s ability to stop premature hormonal activation. Studies have shown the compound prevents premature epiphyseal closure and controls inappropriate sexual maturation in experimental models. The gnrh agonist’s ability to suppress gonadotropin release makes it useful for developmental endocrinology research.
Reproductive Biology Research
In assisted reproduction research triptorelin is a key tool for controlling endogenous gonadotropin release during experimental protocols. Research has shown the compound improves cycle control and optimizes experimental conditions for oocyte studies. The substance is also used in hormone responsive cancer research, particularly breast cancer models where estrogen dependent conditions require precise hormonal control.
Studies have also been done on triptorelin in uterine fibroids research where the compound creates a hypoestrogenic state for experimental conditions to study estrogen dependent tissue growth mechanisms.
Storage and Safety Information
Triptorelin comes as a sterile-filtered, freeze dried white powder optimized for research use. The lyophilized form ensures stability during ambient temperature shipping and maintains compound integrity for laboratory use.
Storage Requirements
Long term storage requires desiccated conditions below -18°C to prevent hydrolysis and maintain peptide stability. The lyophilized form is stable at room temperature for up to three weeks, providing flexibility for laboratory handling procedures.
Upon reconstitution in sterile 18MΩ-cm H2O to a minimum concentration of 100 µg/ml prepare a stock solution suitable for experimental dilution required protocols. Reconstituted solutions are stable at 4°C for 2-7 days, though freeze storage extends usability for longer experimental timelines. Avoid repeated freeze-thaw cycles to preserve peptide integrity and ensure consistent experimental results.
Safety Precautions
Research has shown triptorelin is non-mutagenic, non-clastogenic and non-teratogenic in standard laboratory tests. However the compound’s anti-gonadal effects require appropriate handling in research environments. Some studies have noted transient serum enzyme elevations though these are within acceptable ranges for research use.
The compound has negligible protein binding at experimental concentrations and metabolism does not involve hepatic cytochrome P450 enzyme systems. These characteristics contribute to predictable pharmacokinetic behavior in research models.
Why Buy from Loti Labs
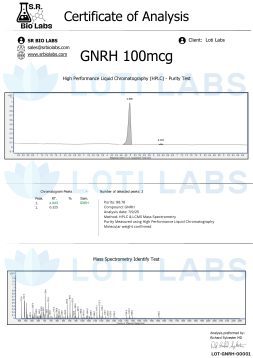
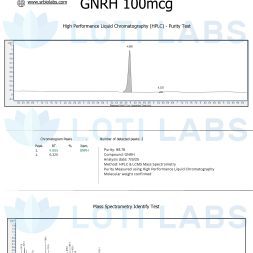
When you buy gnrh triptorelin from Loti Labs you get a compound that meets the highest quality standards for reproducible research. Our triptorelin has a guaranteed minimum purity of >98.0% as verified by third-party HPLC analysis.
Quality Control
Every batch is validated by independent labs to ensure product identity and potency across all research applications. The sterile-filtered and lyophilized preparation has no additives for maximum flexibility in experimental design and custom reconstitution protocols.
Research Benefits
- High Purity: >98.0% purity verified by HPLC
- Consistent Potency: Batch-tested for reliable experimental results
- Flexible Formulation: Additive-free preparation for custom protocols
- Bulk Options: Cost-effective for large-scale research programs
- Documentation: Complete batch records for regulatory compliance
The compound is available as acetate or pamoate salts to suit your experimental needs. Whether you are investigating diagnostic or therapeutic procedures in lab models or conducting fundamental hormone research our triptorelin meets the exacting standards for scientific progress.
Research Use Only
All products from Loti Labs are designated for research use only and in-vitro laboratory testing. This designation ensures compliance with regulations while providing researchers access to high quality compounds for scientific research. The triptorelin compound is not for human or veterinary use and cannot be used outside of a controlled lab environment.
Compliance
Researchers must follow all handling procedures according to institutional safety protocols and relevant regulations. The substance cannot be used as food, cosmetic or for any use beyond laboratory research. This research use designation protects both the investigator and the institution while allowing legitimate scientific inquiry.
Legal
The compound requires proper documentation and handling according to research chemical protocols. Investigators must keep records and ensure all experimental procedures are in compliance with institutional oversight.
Shipping and Customer Service
Expedited Shipping
Loti Labs ships same day for orders placed before 1pm EST Monday-Friday. Orders placed after 1pm EST or on weekends ship the following business day to minimize research downtime.
Satisfaction Guarantee
Our 30-day satisfaction guarantee allows returns of unopened products for full refund, reducing the risk for research institutions. This policy shows we stand behind our product and provides researchers with purchase security.
Customer Support
Our technical support team can help with product specifications, storage recommendations and experimental considerations to ensure you get the most out of your research materials and have any questions about product handling or use.
Third Party Testing and Quality Verification
Every batch of triptorelin is tested by independent labs for identity and purity >98.0% to ensure the main peptide peak is free of significant contaminants that could impact experimental validity.
Documentation
Complete batch documentation is included with every shipment, providing you with all the analytical data you need for regulatory compliance and experimental validation. This includes chromatographic data, purity specifications and storage recommendations.
Quality
Our purity meets the strict standards for research chemicals, recognizing that even small variations in peptide composition can affect experimental reproducibility and result interpretation. The >98.0% purity ensures you get a compound suitable for the most demanding scientific applications.
GnRH Agonist Research
The field of gonadotropin releasing hormone research is expanding as researchers explore the compound’s applications across multiple research areas. From fundamental endocrinology studies to complex oncology research, triptorelin is a tool for investigating hormone dependent mechanisms.
Research shows that the first few weeks of triptorelin exposure are critical for experimental design as the compound has a biphasic response pattern that affects experimental outcomes. The initial stimulation followed by suppression provides opportunities for various research applications from acute hormone studies to chronic suppression protocols.
Studies comparing triptorelin to other gnrh agonists show subtle differences in onset and potency that may impact experimental design choices. While most common side effects in research models are related to expected hormonal changes, investigators should consider these when designing experimental protocols.
The compound has applications beyond traditional endocrinology research with emerging studies on metabolic changes, behavioral research including gender identity disorder models and potential interactions with selective serotonin reuptake inhibitors in complex experimental paradigms.
For researchers looking for locally advanced expertise in hormone research, triptorelin is a key compound to investigate fundamental biological processes. Whether you are studying the basic mechanisms of lh rh action or exploring complex hormone dependent pathways, this gnrh agonist provides the reliability and potency to move your science forward.GnRH research is evolving and requires high quality research compounds that meet the standards of modern scientific research. When you buy gnrh triptorelin from Loti Labs you get a compound that meets the standards of contemporary hormone research and compliance for legitimate research.
References
- Abbara A, Phylactou M, Eng PC, et al. Endocrine responses to triptorelin in healthy women, women with Polycystic Ovary Syndrome and Hypothalamic Amenorrhea. J Clin Endocrinol Metab. 2023;dgad026. doi:10.1210/clinem/dgad026
- Zhou Y, Jia R, Kong FS, et al. A single blood sample for stimulated LH assayed by ICMA is useful for monitoring the treatment efficacy of triptorelin depot in girls. Scand J Clin Lab Invest. 2022;82(7-8):588-594. doi:10.1080/00365513.2022.2148120
- García García E, Álvarez Del Vayo C, Castaño L. Familial male-limited precocious puberty (testotoxicosis): Usefulness of treatment with ketoconazole and triptorelin. Med Clin (Barc). 2022;159(11):e79-e80. doi:10.1016/j.medcli.2022.08.007
- Li X, Li H, Shi H, et al. Assessment of Two Formulations of Triptorelin in Chinese Patients with Endometriosis: A Phase 3, Randomized Controlled Trial. Adv Ther. 2022;39(10):4663-4677. doi:10.1007/s12325-022-02264-5
- Uzonwanne VO, Navabi A, Obayemi JD, et al. Triptorelin-functionalized PEG-coated biosynthesized gold nanoparticles: Effects of receptor-ligand interactions on adhesion to triple negative breast cancer cells. Biomater Adv. 2022;136:212801. doi:10.1016/j.bioadv.2022.2128016. Metbulut AP, Adıgüzel KT, İslamoğlu C, et al. Hypersensitivity reactions with leuprolide acetate and triptorelin acetate in children. Indian J Endocrinol Metab. 2021;25(6):527-531. doi:10.4103/ijem.ijem_333_21
- Zhu L, Guan Z, Huang Y, et al. Triptorelin therapy after conservative surgery for deep infiltrating endometriosis: A multicenter, prospective, non-interventional study in China. Medicine (Baltimore). 2022;101(5):e28766. doi:10.1097/MD.0000000000028766
- Nikzad F, Sadjadi SA, Mohammadpour AH, et al. Triptorelin as an adjunct in treatment of obsessive-compulsive disorder in patients receiving selective serotonin reuptake inhibitors (SSRIs). Iran J Psychiatry. 2021;16(4):444-450. doi:10.18502/ijps.v16i4.7232
- Lu X, Sun Y, Han M, et al. Silk fibroin double-layer microneedles for encapsulation and controlled release of triptorelin. Int J Pharm. 2022;613:121433. doi:10.1016/j.ijpharm.2021.121433
- Vukovic R, Milenkovic T, Soldatovic I, et al. Triptorelin stimulated luteinizing hormone concentrations for diagnosing central precocious puberty: diagnostic accuracy study. Endocrine. 2022;75(3):934-941. doi:10.1007/s12020-021-02947-z
For more information on GnRH Triptorelin please visit Wikipedia.
| Weight | 0.0099 lbs |
| Appearance | Fine White Lyophilized Powder |
| Residue Sequence | pGlu-His-Trp-Ser-Tyr-D-Trp-Leu-Arg-Pro-Gly-NH2 |
| Solubility | 100 µg/mL sterile diluent (distilled de-ionized water) |
| Source | Biosynthetic production |
| Stability | Lyophilized protein is to be stored at -20°C. It is recommended to divide the remaining reconstituted peptide into multiple vials so as to avoid a cycle of freezing and thawing. Reconstituted protein can be stored at 4°C. |
| Molar Mass | 1311.45 g/mol |
| CAS Number | 57773-63-4 |
| PubChem | CID 25077179 |
| Molecular Formula | C64H82N18O13 |
| MG | 100mcg |
| Terms | This product is sold for research/laboratory usage only. No other uses are permited. |
| Weight | .03125 lbs |
|---|